Eugene S. Takle
© 1996
Today we will start a two-part series on man-made chemicals with long atmospheric lifetimes and the atmospheric and chemical processes relating to global change. We begin with a summary of ozone observations and its reactions in the stratosphere. NASA has provided a glossary of terms relating to ozone and CFC's and information on understanding ozone.
The first graph is the first published evidence of major ozone depletion over Antarctica.
 |
First published evidence of ozone depletion. Rowland, 1989: American Scientist 77, 36. Permission granted by Sigma, Xi, The Scientific Research Society. |
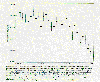
This peculiar behavior in ozone concentrations was not limited to Antarctica, but the effect was most pronounced in that region. The accompanying graph gives observations from Switzerland plotted as the difference in average ozone levels between the period 1931-1969 and 1970-1986.

|
Evidence of ozone depletion outside Antarctica has not been easy to obtain. Rowland, 1989: American Scientist, 77, 36. Permission granted by Sigma, Xi, The Scientific Research Society. |
The next figure shows data taken more recently from a vertical profile of partial pressure of ozone, in nanobars, as a function of height.

| South Pole Station, 1992 Ozone Hole. E. Dutton, NOAA. |
Reactions that destroy ozone are shown in the next figure.

|
CFC reaction equations. |
So if chlorine (along with NO and OH, which will be considered later) causes ozone destruction in the stratosphere, where does it come from, how does it get there, and why is there more of a problem now that 50 years ago? The major source of free chlorine at the earth's surface is sea salt, NaCl, which can dissociate and provide an abundant supply of chlorine atoms over the global oceans. While this represents a very large natural source of free chlorine, only a tiny fraction survives to reach the stratosphere. The amount that does escape from the troposphere contributes natural destruction of ozone that balances natural creation by solar radiation.
Anthropogenic sources of chlorine include industrial and household processes using chlorine for cleaning, disinfecting, and bleaches. Chlorine also is widely used in water supplies and swimming pools.
| Discussion with Don Wuebbles concerning the phase out of chlorine use in industry | Note: You must first click the "view" icon after transferring to the electronic dialog page before the material can be accessed. |

|
Distribution of Chlorine in the normal stratosphere. |
Chlorine-containing molecules, such as the CFC's, CF2, CL2, CCl3F, that have long enough lifetimes will diffuse to the stratosphere where they are acted on by ultraviolet radiation.

|
Vertical distribution of chlorine compounds in the atmosphere. |
To answer these questions, we need to understand a little about the meteorology and chemistry of the stratosphere in the Southern Hemisphere. In July the South Pole has the polar night, or polar winter, so the sun remains below the horizon all day and the whole atmosphere from the surface to the lower stratosphere gets very cold. Temperatures in the stratosphere drop to -90 degrees Celsius and clouds of ice particles begin to form. The ice crystals in these clouds provide a local surface for heterogeneous chemistry to take place.

|
Stratospheric chemistry (simplified). Takle, G.S., 1995 |

|
Polar Stratospheric
Meteorology. Takle, G.S., 1995
|
NASA scientists have clearly demonstrated this connection by flying through these clouds, measuring concentrations of chlorine, chlorine nitrate, and ozone. The chlorine - chlorine nitrate - ozone depletion linkage is very well established. Three stratospheric chemists have won the Nobel prize in chemistry for their work in clarifying these conditions. Despite this overwhelming scientific evidence for the linkage between increasing concentrations of CFCs and ozone depletion, articles continue to appear in otherwise very respectable business magazines denying this connection. Such articles typically cite some scientist with no published work in this area as their authorities. This is a clear case of the need to carefully and critically review the evidence for claims that go counter to the scientific consensus.
The next table gives the lifetimes of different chlorine-containing compounds.

|
Major Halocarbons: Statistics and Uses. Sources: EPA, 1988a; Hammitt, et al., 1987; Wuebbles, 1983,; WMO, 1985. |
It is possible to reduce the adverse effects of CFCs by adding a hydrogen atom to the molecular structure. This makes the molecule somewhat more reactive and less likely to survive long enough to diffuse to the stratosphere. The resulting molecule is called an HCFC or HFC. For example, HFC-134a is a possible replacement for CFC-12, but requires a much more complicated process for production, as is shown in the accompanying figure.

|
Production of HFC-134a. Source unknown. |
Transcription by Theresa M. Nichols