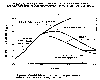1-9: Ozone Reactions: Physics and Chemistry of the Stratosphere
Eugene S. Takle
© 1998
The CFCs present a double danger to the global environment because
they have the potential for both ozone depletion and global warming, since
they also are greenhouse gases. The next graph shows the energy absorbing
potential (band strength) for various gases at different wavelengths.

|
Infrared radiation window.
Source unknown.
|
Infrared radiation from the earth has substantial energy at wavelengths
between 7 and 10 micrometers. The graph shows that the CFCs (underlined in
blue) have moderate to high absorbing capabilities at these wavelengths and
thereby contribute to global warming.
The next figure gives the combined global warming and ozone
depleting effects of the chlorinated compounds.

|
Double danger from CFCs.
EPRI Journal, September, 1989.
|
Molecules located in the
upper right part of the chart have large detrimental effects for both
reasons and are candidates to be replaced with more benign molecules
located in the lower left part of the chart. The process of making an
HCFC from a CFC reduces the environmental impact of the molecule but it
negates one of the reasons the CFCs were created in the first place, namely
because they are so non-reactive. The result is that when HCFCs are used
in, say, a refrigerator they also interacts with the lubricating oil added
to the working fluid. This means that the lubricating capability is
diminished, which reduces the lifetime of the refrigerator's compressor.
Also, the compressor may require a higher operating pressure which demands
more energy and puts more strain on seals and other components, making the
refrigerator less energy efficient and subject to malfunction. The CFCs
are very convenient chemicals, except for their environmental hazard, and
several negative factors that must be considered in the search for suitable
replacements. The Upper Atmosphere Research Satellite (UARS) measures temperature, pressure, wind
variability and gas species concentrations in the stratosphere at altitude ranges from 10 to 100 km.
The present trends in atmospheric concentration are shown in the
next figure.

|
Atmospheric concentrations
and trends of halocarbons. Adapted from IPCC, 1992, 38.
|
Note that concentrations are given in parts per trillion per
year (ppt/yr). The most recent data reveal that the concentrations are
still increasing but less rapidly than in the past.
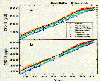
|
CFC-11 and CFC-12
concentrations from 1978-1992. Dutton, E., NOAA (published in
Nature by Elkins et al, 1992).
|
Concentrations will
likely increase for some time for several reasons. Although the CFCs are
not manufactured in the US, they are manufactured in some countries and are
even the substance of a developing black market for international trade.
Also, CFCs used before the manufacturing ban remain in air conditioners, in
automobiles, homes and commercial and industrial buildings as well as in
junkyards and landfills. As equipment containing CFCs deteriorate, seals
age and containers rupture releasing CFCs to the atmosphere for some time.
CFCs have been used in the past for manufacturing foams that are used for
insulating materials. These foams have millions of tiny bubbles containing
CFCs, which do not readily diffuse through the foam, retain their
insulating property. Eventually, however, they will be released to the
atmosphere, creating a source of atmospheric CFCs long after they are no
longer manufactured.
There is some encouraging news however, on atmospheric CFC
concentrations: even though measurements are consistent in showing
continued increases in CFC concentrations, they are increasing at a
decreasing rate, as shown in the accompanying graph for Alaska, Colorado,
Hawaii, Samoa, and the South Pole.
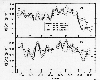
|
Annual growth rates of CFC-11 and CFC-12 from 1978-1992. Elkins, et al, 1992:
Nature.
|
For example CFC 11 presently is
increasing at only about four parts per trillion per year as compared with
about 10 to 12 ppt just a few years ago. The
1989 Montreal Protocol to
Reduce Substances that Deplete the Ozone Layer (and subsequent amendments)
called for the elimination of CFC-11, -12, -113, -114, and -115 and methyl
chloroform (CH3Cl3), carbon tetrachloride (CCl4) and the halons (H-1211,
- -1301, -2402) by the end of the century. This is widely regarded as the
stimulating motivation for major international agreement on a significant
environmental issue. The
CFC numbering scheme was devised to label these molecules without revealing their molecular structure.
In the section on human health, we will discuss in more detail the
reason stratospheric ozone is directly important to humans, but at this
point we will briefly discuss the relationship of stratospheric ozone to
ultraviolet (UV) light.

|
Calculated UV spectrum of solar energy entering the atmosphere... (after C and E News.
Nov24, 1986)
|
Wavelengths of light in the visible part of the
spectrum of solar energy extend from about 0.7 to about 0.4 nanometers.
Radiant energy having wavelengths shorter than about 0.4 nanometers is
classified as ultraviolet light, "near UV" or UV-A for wavelength of 400 to
320 nanometers, mid UV or UV-B for wavelengths of 320 to 290 nanometers,
and UV-C for wavelengths of 290 to 250 nanometers. Stratospheric ozone is
very efficient at absorbing solar energy with wavelengths of less than 290
nanometers, but its efficiency drops off in the UV-B range (the range of
wavelengths that give a sunburn).

|
Change in biologically damaging
UV flux with change in ozone concentration...(after C and E News, Nov 24,
1986. )
|
As stratospheric ozone is depleted, more
energy in the UV-B range is allowed to pass though the stratosphere and
troposphere and enter the biosphere - the sphere of plant, animal, marine,
soil organisms near the earth's surface.
Some evidence from Canada in the accompanying graph shows UV
radiation at the earth's surface increasing differently at different
wavelengths, but the largest observed increase is at those wavelengths
where ozone absorption is large.

|
Spectral distribution of ozone absorption and UV
increase. (Adapted from Kerr and McElroy, 1993: Science,
Vol 262, 1032.)
|
Fortunately the increase is less in the
summer, when humans have more skin exposed to the sun, than it is in the
winter. The NOAA/EPA UV index provides a measure of skin damage caused by UV radiation based on weather
forecasts.

|
Spectral
distribution of average daily total UV-B solar energy flux. (adapted from Kerr and
McElroy, Science, 262, 1034, 1993)
|
Additional data from Montreal shows that the average daily flux
of UV-B radiation is somewhat higher in 1993 that 1989 for both summer and
winter. Updates on current ozone measurements from Antarctica can be obtained from the stations at Halley,
Rothera and Vernadsky/Faraday. Canadian monthly and annual graphs of ozone levels
demonstrate that ozone levels over several Canadian cities fall typically 5-10% below normal levels in the winter
months.
Regional average ozone decreases for other areas from 1960 to 1990
show weakly declining values over North America, Europe, and the Far East.

|
Regional average ozone depletion. Science. Vol.
256, Apr. 17 ,1992.
|
High latitudes generally are experiencing more rapid decreases, and
decreases in winter are larger than in summer.
Ozone depletion is related to increases in skin cancer. A
depletion of 2% total ozone is expected to lead to about one-half million
additional cases of skin cancer and additional 9,300 deaths. Measured
values of ozone in the latitude range of the United States currently are
about 6-7% below the natural levels.
There is some encouraging news in the long term as can be seen in
the next graph which shows the atmospheric concentration of chlorine from
1960 to the present and projected into the future.
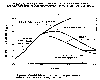
|
Measured (1960-1990) and
projected atmospheric concentrations of chlorine. (After C & E News,
May 24, 1993)
|
The first strong
evidence for the existence of the Antarctic ozone hole was in the 1970's
when chlorine concentrations reached about 2 ppb. When the CFCs were
implicated as the source of the chlorine leading to ozone destruction the
1987 Montreal Protocol was adopted. As confirming evidence continued to
accumulate, the 1990 and 1992 revisions put increased restrictions on CFC
production. The graph shows that with the present restrictions in place
ozone levels may return to 1970 levels by the year 2060. DuPont Chemical
which was a major producer of CFCs, and the one that invented them in the
first place, was very quick to abandon their production of CFCs.
 |
Summary |
Transcription by Theresa M. Nichols